
Marya GMP Certified Vial Liquid Washing Drying Filling Stoppering Production Line for Pharmaceutical Manufacturer Supplier
Basic Info.
| Model NO. | MY-VIAL |
| Certification | ISO, CE |
| Automation | Automation |
| Flexible Production | Intelligent Manufacturing |
| Rhythm | Flow Production Line |
| Production Scope | Product Line |
| After-sales Service | Field Installation, Commissioning and Training |
| Dosing Device | Plunger |
| Feed Cylinder Structure | Single-Room Feeding |
| Material Type | Liquid |
| Packaging Container Lifting Structure | Pneumatic Lifting |
| Filling Valve Head | Multi-Head |
| Packaging Material | Glass |
| Type | Piston Filling Machine |
| Filling Principle | Pressure |
| Structure | Linear |
| Transport Package | Inner Water Proof Bag, Outer Sea Worth Case |
| Specification | Customized |
| Trademark | Marya |
| Origin | China |
| HS Code | 842230109 |
| Production Capacity | 1000 Set/Year |
Packaging & Delivery
Package Size 350.00cm * 150.00cm * 180.00cm Package Gross Weight 3500.000kgProduct Description
Marya GMP Certified Vial Liquid Washing Drying Filling Stoppering Production Line for Pharmaceutical Manufacturer SupplierProduct Description
Main Application:The vial filling production line is composed of bottle unscrambler, rough washing machine, fine washing machine, filling and stoppering machine, capping machine. It can complete bottle unscrambling, rough washing, fine washing, nitrogen filling, vacuuming, stopper unscrambling, stopper pressing, cap unscrambling, capping and other complex functions, realizing automatic production of the whole process. Each machine can be used separately or in linkage line. The whole line is mainly used for production of sterile glass bottle IV infusions, and also final sterilized drugs.
Technical Parameters:
| Model | KGF4 | KGF6 | KGF8 | KGF10 | KGF12 | KGF20 | KGF24 |
| Applicable specifications | 2~30ml vial bottles | ||||||
| Filling heads | 4 | 6 | 8 | 10 | 12 | 20 | 24 |
| Production capacity | 50-100bts/min | 80-150bts/min | 100-200bts/min | 150-300bts/min | 200-400bts/min | 250-500bts/min | 300-600bts/min |
| Stoppling qualification rate | >=99% | ||||||
| Laminar air cleanliness | 100 grade | ||||||
| Vacuum pumping speed | 10m3/h | 30m3/h | 50m3/h | 60m3/h | 60m3/h | 100m3/h | 120m3/h |
| Power consumption | 5kw | ||||||
| Power supply | 380V 50Hz | ||||||
Performance Features:
1. The whole line meets new GMP requirements, and the cleaning effect meets the new Pharmacopoeia standards and requirements.
2. The whole vial filling production line can be designed in diversified layout according to plant site, to reduce risk of drug cross-contamination, and ensure convenience of operation of personnel and materials.
3. Vial filling line applicable specification: 50ml-1000ml iv glass bottle (as per user's requirement)
4. Production Capacity of vial filling machine: 1000-21000BPH
5. Number of filling heads: 1-20, to be selected according to output6. Vial filling machine's filling accuracy: ≤±1% (according to drug characteristics)7. Capping qualified rate: ≥99.9%
8. Compact and simple structure occupies less area;
9. Stable product performance, easy and reliable operation, beautiful appearance;
10. High degree of automation, few operators required;
11. The vial filling machine uses the principle of flow control for filling, the filling volume is adjusted by computer, and the measurement is accurate.12. Filling machine has CIP/SIP function.13. The whole open-RABS isolation protection system and class 100 laminar flow hood protection;14. Optional high-performance no-bottle-no-filling, no bottle no stoppering, no bottle no capping and squeeze stop functions;
15. Full-line linkage control function;
16. The capping machine can be equipped with dust exhaust device, which can absorb aluminum scraps produced during capping and thus reduce the risk of environmental pollution.17. The whole vial filling line can be equipped with online monitoring system to monitor key factors that affect product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.).18. To realize fully automatic control and monitoring of production process, high precision colored touch screen operation monitoring, PLC automatic control & automatic protection, main machine frequency conversion speed regulation and other control technology are used.19. Applicable for wide range of bottle specifications, and easy to replace mould.20. The products can be customized according to the actual demand of customers.
About Us

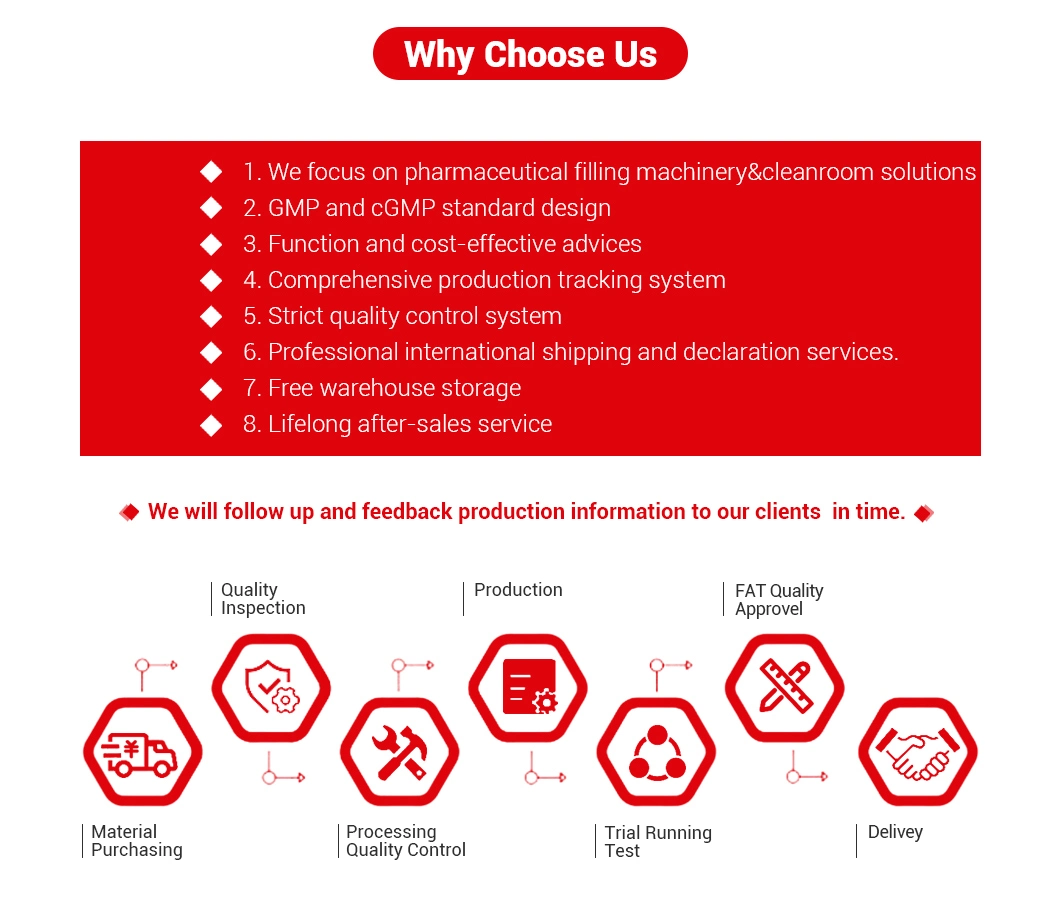
Why choose us
Workshop

Certificate


Project cases & Our Customer
Exhibition & Our Team
Packaging & Shipping






